Anyone trying to lose weight typically focuses on diet and exercise. Those traditional levers, which involve cutting back on food intake and ramping up workouts, are based on the calories-in-calories-out concept of shedding pounds. But there’s a growing awareness that the rudimentary math of this approach misses some critical contributors to successful weight loss. Increasingly, researchers believe that what you eat and its impact on your metabolic health play a more significant role in weight and obesity. And good sleep can be a vital component of this model.
“Increasingly, researchers believe that what you eat and its impact on your metabolic health play a more significant role in weight and obesity. And sleep can be a vital component of this model.”
Sleep is linked to several hormonal and metabolic processes that keep your overall metabolism in a state of homeostasis, or balance. How well you sleep and the number of hours you spend asleep—adults need at least 7 hours a night—can alter your appetite and feelings of hunger as well as your body’s ability to store and use energy, all of which can have a direct effect on your ability to maintain or lose weight. When you don’t get enough sleep or have a sleep disorder such as sleep apnea, it can throw your metabolism off kilter and lead to weight gain and even chronic illness.
How does healthy sleep help orchestrate these metabolic activities that play out in your everyday life, and what are the biggest consequences of poor or inadequate sleep on weight loss? Let’s explore.
Weight Loss: More Than Just Calories In, Calories Out
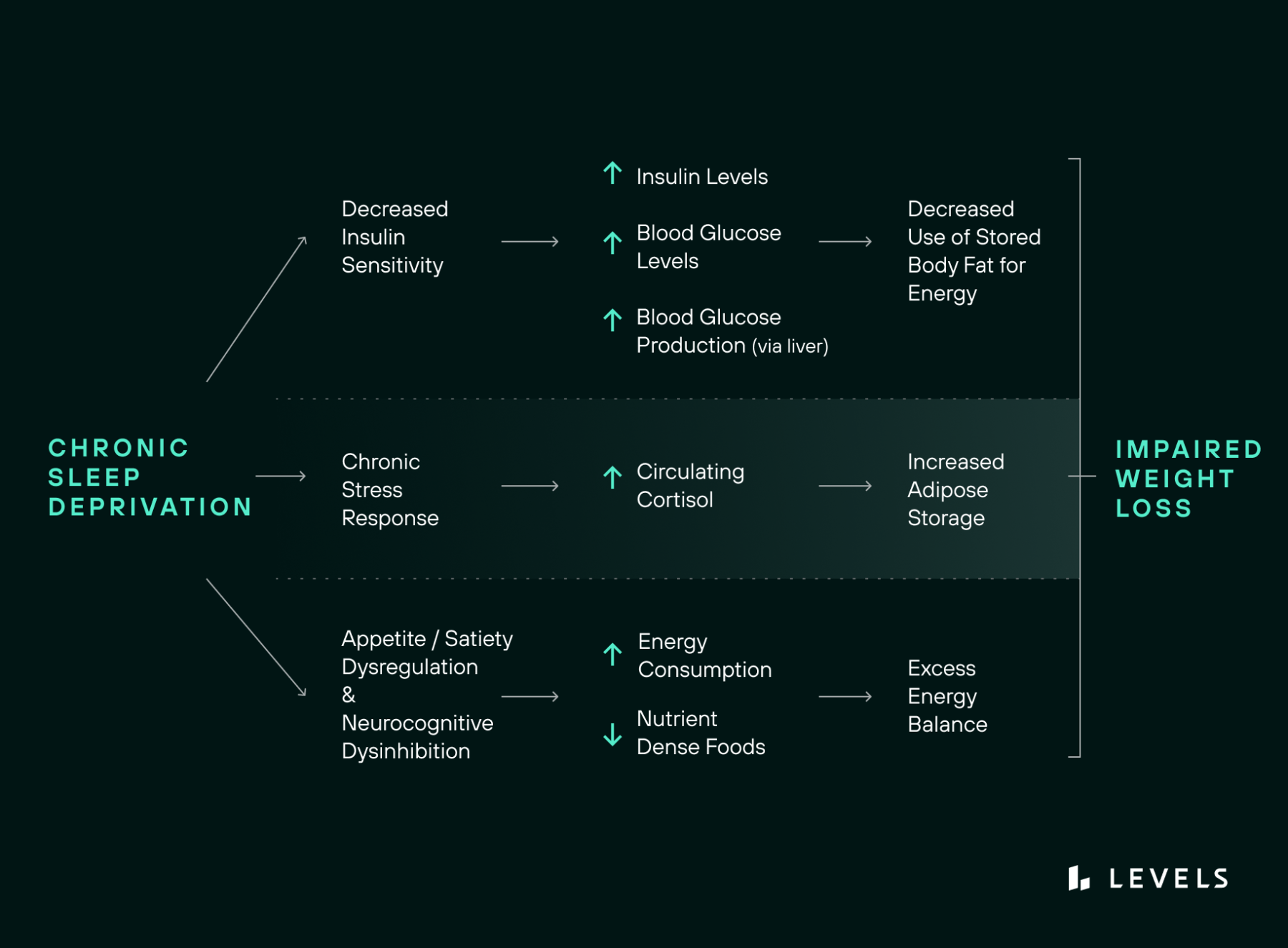
The notion that consuming fewer calories alone will lead to long-term weight loss is faulty. This simple strategy fails to consider the complex workings of energy balance and its role in weight gain and loss. Beyond the number of calories a person takes in, their hormonal responses—and in particular, the insulin response—to the types and timing of food they consume help determine the efficiency with which digested food is processed and whether or not it is stored as fat.
A newly published research review explores this model in great depth and cites dozens of studies demonstrating how consumption of excess carbohydrates (particularly in the form of sugars and refined grains) prompts our bodies to store more fat. Insulin, the body’s blood glucose regulator, is the crucial factor that drives this. It helps ferry glucose into muscle cells to be either burned or stored. It also suppresses glucose production in the liver and aids in maintaining fat stores. Levels of circulating blood glucose trigger the rise and fall of insulin such that when glucose levels are higher than needed for the body’s energy demands, insulin rises to signal the body to use or store glucose, and when glucose levels fall (between meals or during sleep), insulin levels drop too. That lowering of insulin (and increase in glucagon) is the body’s signal to burn stored energy—first glycogen and then fat. One key take-home message: The body won’t use its fat stores for energy while insulin levels are high. And anything that raises blood glucose, such as eating lots of carbs and sugar, will make weight loss harder.
This finely tuned system, critical to maintaining a healthy weight, can get derailed. And high on the list of what can derail it is poor or inadequate sleep.
How Does Sleep Impact Glucose, Insulin, and Metabolism?
Accumulating research confirms that not getting enough sleep affects insulin levels and glucose metabolism in the body.
Even a few nights of poor sleep can lead to higher fasting glucose and reduced insulin sensitivity, which means your cells are less responsive to insulin, requiring your body to produce more of it to process its glucose. In 1999, a small laboratory study found that sleeping just four hours per night for six nights could play havoc with metabolic and endocrine functions by increasing the time it took for blood glucose to be cleared after a high-carb meal, reducing the rate of glucose clearance by 40 percent and the acute insulin response to glucose by 30 percent.
Similar results have since been found in studies of both men and women. In one, at the Leiden University Medical Center, even a single night of four hours of sleep reduced insulin sensitivity in healthy men and women by 19-25 percent along multiple metabolic pathways. Another recent study suggests that sleep quality is also essential. Researchers found that poor sleep quality and going to bed later were linked to higher blood glucose levels and worse blood sugar control after meals the next day. (In this study, modest reductions in sleep duration did not lead to adverse effects on glucose control, and the researchers speculate that short sleep duration affects glucose negatively only at very low levels.)
Large epidemiologic studies have repeatedly found that people who are chronically sleep-deprived are at higher risk of obesity.
Why does lack of sleep make your body less sensitive to insulin and less efficient at metabolizing blood glucose? One culprit may be a reduction in slow-wave sleep. Human sleep comprises rapid-eye-movement (REM) sleep and stages 1-3 of non-REM sleep. The deepest stage (3) of non-REM sleep, known as slow-wave sleep, is considered the most restorative. During slow-wave sleep, there are transient metabolic, hormonal, and neurophysiologic changes, including lowered use of glucose by the brain, stimulation of growth hormone release, and decreased sympathetic nervous activity. In one study, suppressing slow-wave sleep for three nights while keeping total sleep time unchanged resulted in a decrease in insulin sensitivity and glucose tolerance—similar to the range seen in older adults with impaired glucose tolerance. And the more significant the reduction in slow-wave sleep, the greater the decrease in insulin sensitivity.
Other likely factors caused by sleep deprivation disrupt insulin and glucose metabolism, including increased sympathetic nervous system activity, which can boost insulin resistance both directly in your tissues and indirectly through the release of pro-inflammatory cytokines (immune system components that can interfere with insulin signaling). As little as one night of reduced sleep can increase cytokine release. And shortened sleep causes the brain—which generally uses up to 20 percent of the body’s glucose while at rest (although it’s just 2 percent of our body weight)—to use less, which leaves more glucose circulating in your blood.
Learn More:
Sleep duration may also affect whether your body burns fat or muscle while on a diet. In one study, people sleeping 5½ hours a night lost more non-fat mass than fat while on a mildly calorie-restricted diet; people who got 8½ hours of sleep shed more fat. The reason may be that the short sleepers also had higher levels of acylated ghrelin, a hunger-promoting hormone (see below) that encourages fat retention and glucose production by the liver. The authors suggest that the lack of sleep changed the way a person’s body uses different kinds of fuel, with short sleepers converting protein to glucose to meet their body’s energy needs. In another study, among people who reduced their calories by 600-700 a day, every extra hour of sleep was associated with 1½ pounds more fat loss.
New research from Mayo Clinic also connects sleep loss to the accumulation of dangerous abdominal fat specifically. In the study, 12 people were subjected to sleep deprivation (4 hours of sleep) for two weeks. Researchers observed an 11 percent increase in visceral fat accumulation inside the abdomen when subjects were sleep-deprived compared to getting a full nine hours (meanwhile, the average weight gain during sleep deprivation was one pound more than under control conditions).
Functional medicine doctor Rich Joseph points out that sleep deprivation has acute and chronic effects. Some consequences, such as reduced insulin sensitivity, may be transitory, while the longer-term impacts on weight gain and metabolic disruption may result from chronic sleep loss.
Summary: Sleep deprivation (consistently less than 7 hours nightly for the average adult) decreases insulin sensitivity (increases insulin resistance) and hence, increases circulating insulin and blood glucose levels.
The Effects of Sleep on Weight Gain, Eating Behaviors, and Food Choices
Sleeping poorly or disrupted sleep patterns can profoundly affect your dietary choices, leading to higher caloric intake and overconsumption of carbs and sugar the next day. A 2019 meta-analysis found that compared to normal sleepers, short sleepers were hungrier, ate on average 250 more calories over the course of a day, and put on weight. In one study, for instance, people who slept 4½ hours a night for four nights had higher levels of hunger-sparking ghrelin the following days and after each meal. As a result, they consumed roughly 340 calories more—including more carbs and more sweet and salty snacks—than when they slept for 8.5 hours. Elevated evening ghrelin levels seemed to be linked to the short sleepers eating more sweets.
In studies where the amount of sleep is restricted, and food is freely available, people tend to consume far more calories than they need to stay awake. Having more hours to eat is one explanation, but research also points to the deactivation of brain networks involved in cognitive control and the activation of those involved in reward. In one study, sleep restriction reduced neural activity in three cortical brain regions that are needed to optimally evaluate food stimuli when people reported wanting food while also boosting neural activity in a subcortical region (the amygdala) that exaggerates the desirability of foods. In the same study, sleep deprivation also caused cravings for high-calorie snacks and junk food that promote weight gain. In addition, the more sleep-deprived the participants felt, the more of these foods they desired—even though they were already consuming extra calories and didn’t report feeling any hungrier than when they got adequate sleep. This suggests, the researchers note, that sleep loss, as opposed to any metabolic need or hunger, produced these changes.
Lack of sleep may also cause people to eat at a time of day that goes against their biology. In one study, when people slept for only five hours a night and could eat whatever they wanted, they ate more carbs during the day and took in 42 percent more calories as after-dinner snacks, consuming more calories after dinner than during any other individual meal. Nighttime snacking can be especially metabolically challenging. As bedtime approaches, the body’s insulin levels decrease, and the sleep hormone, melatonin, naturally rises. Staying up late can also push melatonin onset later, throwing off the 24-hour circadian clock, which may lead to eating more food at night.
Epidemiologic cohort studies have repeatedly found that people who are chronically sleep-deprived are at higher risk of obesity, likely for all the hormonal and metabolic shifts discussed here. (In that meta-analysis of studies, the risk of obesity went up 9% for every 1 hour fewer of sleep from a baseline of 7-8 hours.) And the more limited the sleep duration, the greater the risk. Similarly, studies of hundreds of people that have examined the relationship between curtailed sleep and Type 2 diabetes have found that those who sleep 5-6 hours a day have double the odds of prediabetes and Type 2 diabetes compared with those who get 7-8 hours a day. In some studies, short sleep was a more significant risk factor than lack of physical activity. But there’s also evidence that the risk may be reversible: In a study of short sleepers who received personalized tips for sleep improvement, they increased their slumber time from 5.6 to 7.1 hours and experienced a significant decrease in overall appetite and their desire for sweet and salty foods over two weeks.
Other Hormonal and Metabolic Changes That Affect Weight
As the research above indicates, weight loss is affected by a complex interplay of hormones, and a number of these are affected by sleep. Here is more detail about other metabolic factors that contribute to body fat storage, fat burning, hunger, appetite, and satiety—which all adjust depending on how well you sleep.
- Cortisol, the body’s primary stress hormone, manages your body’s use of macronutrients, increases blood sugar, and controls your circadian rhythm, among other activities. Sleep deprivation leads to increased cortisol production, which can increase fat storage under certain conditions and decrease insulin sensitivity the following day.
- Growth hormone (GH) has a key role in the regulation of glucose, the breakdown of fats, and protein synthesis. GH is secreted during non-REM sleep and appears to be critical in maintaining our sleep-wake cycle.
- Leptin, a hormone primarily secreted by fat cells, is thought to promote satiety and inhibit appetite. Along with ghrelin, leptin keeps your appetite in balance. Research shows that leptin levels tend to be reduced with sleep deprivation even when there is no change in activity levels or food intake. Insufficient sleep seems to change leptin’s ability to respond appropriately to the body’s energy balance and signal satiety when energy needs have been met.
- Ghrelin is a hunger-promoting hormone that’s released primarily from the stomach. It has the opposite effect of leptin, increasing appetite and food intake. Circulating levels of ghrelin are naturally suppressed after eating and then rebound within a couple of hours, triggering a rise in hunger. Sleep loss of two to three hours a night (at least within typical sleep ranges, i.e., from 9 to 6 hours) can lead to a rise in ghrelin and a drop in leptin, leading to increases in appetite and hunger and cravings for sweet, salty, and starchy—i.e., calorie-dense—foods.
- Orexins, also known as hypocretins, are neuropeptide hormones produced in the brain’s hypothalamus that are involved in wakefulness and energy balance. Orexins become more active in response to decreased leptin and glucose and increased ghrelin, increasing your food intake and energy expenditure. Orexins also appear to inhibit the release of growth hormone.
Summary: Chronic sleep loss can also disrupt the balance of appetite and satiety signaling hormones and neurocognitive processes that influence your eating behaviors, which can result in cravings for ultra palatable foods, increased caloric consumption, and resultant weight gain.
How to Improve Your Sleep for Optimal Weight Loss
We all need sleep—it’s a biological necessity—but optimal sleep is a learned behavior. Take these steps to improve your sleep hygiene to support a healthy weight.
Aim for at least seven hours of sleep per night. Keep in mind that seven hours is the minimum recommended by sleep researchers; you may need more than that for your best health.
Set a regular bedtime and wake-up time—and resolve to stick with them. Inconsistent sleep timing throughout the week can raise insulin resistance. Plus, trying to make up for lack of sleep during the workweek by sleeping in over the weekend can result in loading up on after-dinner snacks, gaining weight, and experiencing reduced insulin sensitivity. And recent research suggests that post–weight loss, having a consistent bedtime may help you keep the pounds and fat off.
Avoid eating dinner close to bedtime, and don’t snack after your evening meal. Accumulating research suggests that finishing calorie intake for the night between 6 p.m. and 8 p.m. is better for your metabolic health, according to Dorothy Sears, PhD, professor of nutrition at Arizona State University. This gives your body time to store nutrients before circadian-controlled insulin action is impaired, in part, by the evening rise in melatonin.
Keep in mind that what you eat can also affect sleep. Caffeine, alcohol, and spicy foods are known sleep disruptors. Research suggests that insomnia triggers may also include large amounts of added sugar and processed grains. Having more vegetables and fruit may act as an antidote.
Create a daily “light routine.” Your sleep habits are affected by sunlight and darkness. Go outdoors to get some sunshine as soon as possible after you awaken. In the evening, dim the lights up to two hours before bedtime, and turn off all electronics (phones, tablets, computers) to encourage parasympathetic activation and sleep onset.
Stop vigorous exercise an hour before bedtime. Exercise, even during the evening, can have a calming effect and help your mind relax and prepare for sleep. But a vigorous workout too close to turning in may not allow time for your heart rate to slow down, resulting in delayed sleep, poorer sleep quality, and nighttime awakenings.
Set up your bedroom for sleep. Set the temperature to be cool, close the shades/curtains to create a dark environment, and invite your dog to settle down in their own bed.
Mitigate thought- or anxiety-related insomnia. Use tools like journaling, meditation, breathwork, or reading to help calm your mind before bed.
Struggling to sleep? Consider seeing a sleep specialist or coach. Sleep disorders like obstructive sleep apnea are pervasive and can magnify the adverse effects of sleep deprivation, resulting in a vicious cycle of weight gain. A sleep specialist can determine whether you have a disorder and help you address it to improve your health and sleep.









