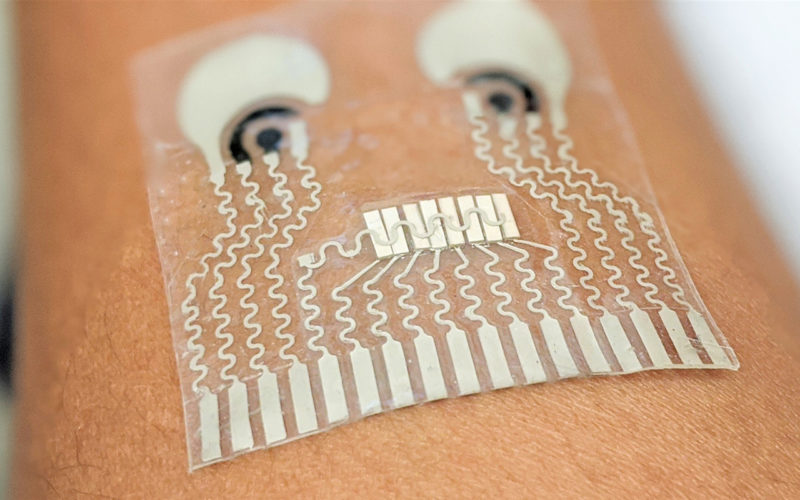
Image credit: Wang lab/UC San Diego
Continuous glucose monitoring is part of a new generation of biowearable technologies that track biomarkers once reserved for medical settings, like heart rate variability and ECG readings, breath rate, hypertension, and ketone levels. Just as the still-growing market of fitness wearables helped people see the value of real-time feedback to exercise, these new biowearables use real-time input to help guide lifestyle decisions and improve overall health.
As this technology becomes more common, the next step is devices worn on or under the skin that track even more cardiovascular and biochemical markers, levels of ingested substances like caffeine, and even infections like COVID-19.
Here’s a look at six technologies in development that show where real-time health monitoring may be headed.
“Next-generation sensors may be smaller, last longer, and read more than glucose.”
The State of Continuous Glucose Monitors Today
The first—and for many, still the current—way to check glucose levels is to prick a finger and put a drop of blood on a test strip inserted into a handheld device, then wait for a reading to appear. About 20 years ago, continuous glucose monitors (CGMs) hit the market to give people with diabetes real-time glucose tracking. Since then, CGM use in Americans with Type 1 diabetes has been growing exponentially, from 6% in 2011 to 38% in 2018, while the price has been falling—the Abbott Freestyle Libre sensor costs $900 to $1800 per year, compared to earlier models that cost $3000 to $5000 annually, while the price of Dexcom’s CGMs has fallen around 40% over the last two model updates. As the CGM market grows among people without diabetes, prices and availability should continue to fall.
How CGMs Work Now
Current models consist of electronics embedded in a plastic disc adhered to the stomach or upper arm. A flexible metal filament around 5mm long and 0.4mm wide pokes into the interstitial fluid surrounding cells just below the skin. Blood glucose continuously seeps from vessels and capillaries into this fluid, where the filament measures it.
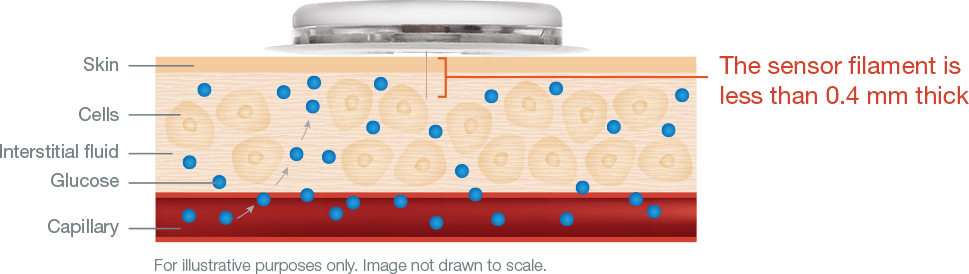
Diagram courtesy Abbott
Coating the filament is an enzyme called glucose oxidase, which converts glucose to hydrogen peroxide. The hydrogen peroxide reacts with platinum to break down into hydrogen, oxygen, and electrons, which generate current. The device wirelessly transmits this signal to a handheld reader or smartphone, which uses an algorithm to convert the electrical signal to a glucose reading. Sensors last for 7 to 14 days before the body’s response around the filament penetration point and degradation of the sensor chemistry lessens the accuracy of the reading.
Next-generation sensors may be smaller, last longer, and read more than glucose.
An Implantable CGM
Beyond patches are implantable devices. In 2019, researchers developed an experimental sensor smaller than a sesame seed placed just below the skin with a custom, user-friendly injector. The key innovation is a novel microchip that measures glucose using the same surface chemistry as other CGMs and sends a wireless signal to a wearable receiver such as a smartwatch. The sensor has a thread that the wearer can pull to remove it when it’s expired.
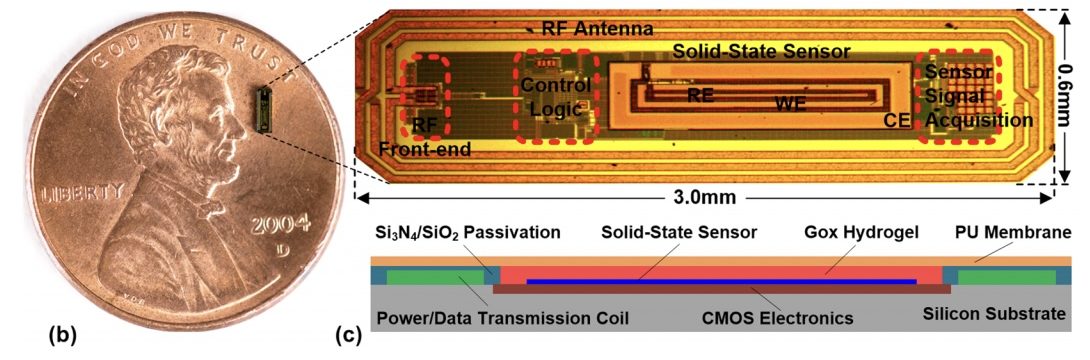
Image and caption source. (b) The Integrated Wireless Sensing Platform on a U.S. one-cent coin. (c) Monolithic sensor including CMOS electronics, solid-state sensor, enzyme hosting hydrogel, and biocompatible membrane.
Shrinking the sensor has several advantages. First, it reduces discomfort and damage to surrounding tissue. Reducing the body’s foreign-body response makes it faster, more accurate, and more reliable. Thin capsules also have a quicker reaction time, as glucose diffuses through it faster. And eliminating wiring reduces noise, increasing accuracy and sensitivity.
The researchers tested their device in pigs, who are good models for human physiology. They injected it below the pigs’ abdominal skin and collected blood samples using an IV line for analysis every 5–10 minutes using a consumer glucose monitor and every 15–20 minutes using a tabletop machine. The sensor started functioning within 10 minutes of implantation and collected data for several weeks. The researchers found that its glucose readings matched those of the other monitors, including after rapid fluctuations in blood glucose. The product is not yet available for sale but points to what’s possible for miniaturization. (Another implantable called Eversense is available now.)
Measuring Insulin and Glucose Together
Different people respond differently to insulin, and the miscalibration of insulin injections presents a constant and potentially deadly risk for people with diabetes. Current sensors can monitor only a few molecules, such as glucose and oxygen, continuously and in real-time. Insulin, which exists at concentrations a billionth that of glucose, remains elusive. But a new device, described in the January issue of Nature Biomedical Engineering, measures both glucose and insulin continuously.
The technology relies on microfluidics—essentially tiny plumbing on a chip 7.5 centimeters long—and microbeads that flow through its pipes. In glucose, blood enters a circuitous pathway called the mixing module, along with microbeads studded with pieces of DNA called aptamers. When the aptamers encounter glucose, they change shape and expose attached molecules called fluorophores, allowing them to glow. The blended fluid passes through a depletion module along with a liquid that washes away the blood cells. Finally, it enters a detection module, where a high-speed camera and software record the concentration of glowing molecules.
Insulin detection follows a similar procedure, except the microbeads are studded with antibodies that bind to insulin, which then binds to other antibodies attached to fluorescent molecules. Unbound antibodies don’t make it through the washing module. The device could potentially measure substances beyond glucose and insulin, such as glucagon, which raises glucose levels, says Mahla Poudineh, an electrical engineer at the University of Waterloo who co-authored the paper during her postdoc at Stanford.
The system is called an RT-ELISA, or real-time ELISA (enzyme-linked immunosorbent assay), and provides signals in only a few seconds. Standard ELISA, a multistep procedure for blood analysis, requires hours. The researchers tested it on rats and found they could detect individual differences in insulin response and differences in response to different insulin formulations. Currently, the system is not available for wearable use (this version was tested through a vein catheter in labs) but could one day be used in clinics for rapid interpretation of blood tests. Poudineh and her collaborators are working on modifying it for continuous wearable monitoring but didn’t comment on specific timing. “That would change lives,” she says.
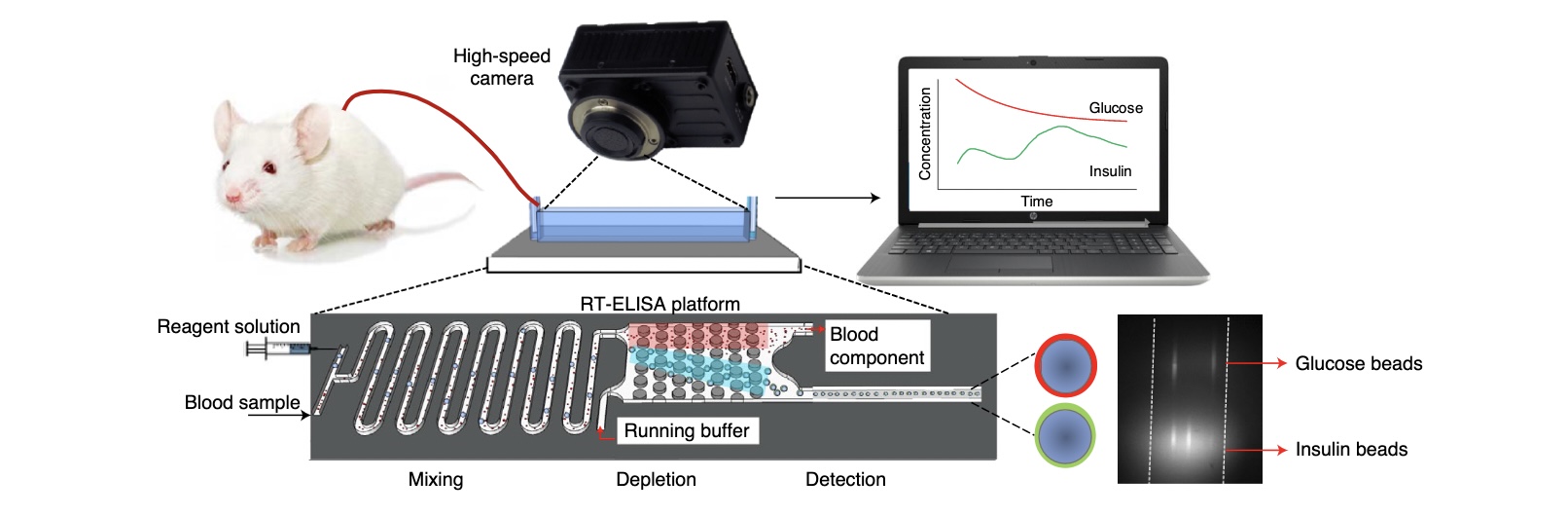
Image and caption source. Overview of the rT-ELiSA technology. The biosensor is connected to a rat through an angio-catheter, with blood injected into the device using a peristaltic pump. The device consists of three modules: a mixing module where blood is combined with the detection reagents (which are introduced to the device via a syringe pump), a depletion module for eliminating excess blood cells, and a detection module that transfers the fluorescently labeled beads to the detection window.
A Patch for Biomarkers and Cardiovascular Monitoring
Taking a complete picture of metabolic health requires monitoring biomarkers in the blood, such as glucose, and cardiovascular performance. Some people with diabetes also have hypertension. Athletes want to measure both hormones and heart rate. Septic shock involves a drop in blood pressure and an increase in blood lactate. But people don’t want to wear multiple devices to measure all of these things. Researchers at the University of California, San Diego have conceived a patch that can monitor many of the above.
The device, a few centimeters across, is a clear, flexible, and stretchable square with visible printed flat wiring and adheres to the neck. It contains two electrodes to draw out bodily fluids for non-invasive sensing: a positive electrode uses electric repulsion to push a drug called pilocarpine into the skin, inducing sweat. From the sweat, one set of sensors measures caffeine, lactate, or alcohol. A negative electrode draws interstitial fluid through the skin and uses the other set of sensors to measure glucose. Currently, the patch can measure two chemicals at a time but could be modified to measure all four. The patch also sends ultrasonic pulses into the artery and measures the echo from the vessel’s near and far walls to track blood pressure and heart rate.
Creating the patch required selecting the suitable materials to make it stretchable and developing integrated chips to reduce power consumption and a design that will further reduce signal interference between components. The team tested it on people in realistic scenarios, asking them to eat food and then exercise or drink alcohol while eating. Its readings matched those of traditional physiological monitors. Currently, the device is not self-powered, but the researchers are working on flexible batteries and renewable energy sources such as solar cells or generators that use friction or sweat, says Lu Yin, a nanoengineering Ph.D. student and paper co-author. “This would open many doors.”
Video originally posted here.
Using Light to Trace Glucose
A more speculative scenario has smartwatches tracking glucose without tapping any bodily fluids. In this concept, a device would shine light through the skin into the blood. When most material absorbs light, it re-emits most at the same wavelength, but some at different wavelengths, a phenomenon called Raman scattering. The brightness of these wavelengths acts as a fingerprint indicating the concentrations of various molecules.
Apple and others are rumored to be developing the technique for future smartwatches, but the technology, which has been studied for years, is just starting to bear out in lab settings.
Using Glucose Meters to Monitor More
Researchers at the University of Toronto developed a kind of molecular translator that takes advantage of existing finger-stick glucose meters to measure additional substances, like SARS-CoV-2 in a patient sample. “We’re leaning on a lot of engineering that’s gone into making the glucose meter so reliable,” says Keith Pardee, a biologist, and paper co-author.
In their system, a patient sample can incite biological reactions that lead to the production of a specific amount of glucose, which can be detected by an off-the-shelf glucose meter. A drop of blood or other fluid sample from a patient is incubated with a short strand of genetic material called RNA. The strand includes a sequence called a toehold switch, which complements the RNA of a particular target you’re looking for in the sample, such as SARS-CoV-2 genetic material. When the RNA strand binds with that target genetic material, it changes shape and exposes the RNA for a reporter enzyme, which is expressed. That enzyme can then convert glucose precursors into single glucose molecules, measurable by a glucose meter. So glucose molecules are only produced if specific genetic material is in the patient sample. Finally, users enter their results into a web interface that translates the glucose score into a score for the target substance.
As a test, the researchers used their method to detect Salmonella Typhi, the bacterium that causes typhoid fever, and SARS-CoV-2, the virus that causes COVID-19. “What was surprising was how stable the results were,” given the variance in most bioassays, Pardee says. The team designed the system for point-of-care use, and they could potentially automate it, says Evan Amalfitano, a Ph.D. student in Pardee’s lab. Scientists might also use it for environmental sensing.
A Continuous Ketone Monitor
The two primary fuels for your body are glucose and fat. When your body burns fat (typically after it has depleted glucose and glycogen), it produces what’s called a ketone body, or ketone. Studies show that using ketones for energy has benefits for muscle development, cell and organ function, and even brain health. Currently, the best way to know whether or not your body is in fat-burning mode is to measure ketones via a breath monitor or a blood-test strip. These methods offer only snapshots, so users can’t see if they are nearing ketosis, or more critically, a dangerous state for people with diabetes called diabetic ketoacidosis.
However, researchers recently demonstrated a continuous ketone monitor (CKM) using a similar form factor and functionality as a continuous glucose monitor. The device, which was tested on 12 healthy people, utilizes a thin filament in subcutaneous interstitial fluid to measure the presence of a kind of ketone called β-hydroxybutyrate (BHB). As in a CGM, the sensor is coated with an enzyme that reacts with the target substance (BHB) such that it produces electrons that can be measured as a signal and then processed and sent to a receiver, like a smartphone. The experimental device is even applied with the same applicator as a CGM.
The challenge to such a system is keeping the sensing chemistry stable for a multi-day wearing period, but in testing, the CKM was more than 90% accurate (within 30% of the results of a blood test strip) over a two-week period. It also showed a quick response, measuring changes in ketone concentrations within four minutes in vitro. The researchers just got funding to try to commercialize the sensors.